Have you ever noticed how some liquids can quickly become gases? This phenomenon is known as vaporization.
Vaporization, or the transition from liquid to gas, is like a magic trick – one moment, you have a liquid; the next, it’s gone. But it’s got nothing to do with smoke and mirrors.
In this article, I will explain what it is called when liquid changes to a gas. You’ll also look at its types and some real-life applications involving vaporization. So, read on to learn more.
The Phenomenon of Liquid Changing to Gas
Vaporization is a fancy word used to describe what happens when a liquid changes into a gas. This can happen when you heat a liquid or when it’s left out in the open air for a while.
When you heat a liquid, the molecules move faster until they gain enough energy to break away from its surface and become a gas. Vaporization is essential in many natural and industrial settings, from the water cycle to chemical processing.
Explanation of the process
Heat energy is typically used to initiate this process, resulting in a rapid change from a liquid or solid to a vapor state.
Have you ever noticed how water can turn into steam when heated? This happens because of a process called vaporization. Imagine you’re at a pool, and the water is very still. You could see the surface of the water. Now imagine you jump in and start swimming.
You make many waves, and the water’s surface becomes less still. If you keep swimming, the water will become choppier and choppier. This is similar to what happens to a liquid when it is heated.
The heat makes the molecules in the liquid move faster and faster, and they bounce around like you are swimming in the pool. When enough energy is added, the molecules break free from the surface of the liquid and become a gas.
This gas is called vapor. This process is important in many daily things, like boiling water to cook pasta or steam engines that use water vapor to move trains.
Vaporization can occur naturally in nature, such as when water changes from a liquid to a gas (water vapor) during evaporation. It can also be achieved in a laboratory or industrial setting using specialized equipment. Sometimes, vaporization is used to extract the compounds within a liquid or solid material for further processing.
Types of Vaporization
1. Evaporation
Have you ever noticed a puddle of water slowly disappears on a hot day? That’s because of a type of vaporization called evaporation. Evaporation happens when a liquid turns into a gas, but only on the surface of the liquid.
This happens when the heat from the sun or the air causes the liquid molecules to move faster and faster until they break free from the surface of the liquid and become a gas. This gas is called vapor.
Evaporation is important in the water cycle, which helps turn water from oceans and lakes into clouds. It’s also how we get salt from saltwater by letting the water evaporate and leaving it behind.
2. Boiling
Boiling happens when a liquid is heated to a certain temperature, called the boiling point, where the molecules gain enough energy to break free and become a gas.
When you heat the water in a pot, the molecules move faster until they bounce around and collide.
When the water reaches 100°C, the boiling point for water, the molecules gain enough energy to break free from the surface of the liquid and become a gas. Furthermore, it is important to note that each liquid has its unique boiling temperature.
This is the temperature at which the saturation vapor pressure equals the external pressure, also known as the boiling point.
Factors Affecting Vaporization
1. Temperature
As the temperature of the liquid increases, the molecules of the liquid gain more kinetic energy, which increases their ability to break free from the surface of the liquid and become a gas.
The higher the temperature, the more energy the molecules have and the more likely they will evaporate. Because of this, liquids boil at higher temperatures higher up where the air pressure is lower, making it easier for water to evaporate on a hot day.
Therefore, the temperature is crucial in determining the rate and degree of evaporation.
2. Surface area
With an increase in surface area, there is a corresponding increase in the vaporization rate.
This is because a greater surface area allows more vapor molecules to come into contact with other vapor molecules, promoting evaporation.
Additionally, larger surface areas allow for increased exposure to external heat sources, further aiding vaporization.
3. Pressure
When pressure is applied to a liquid, molecules become more crowded and cannot move freely, making it more difficult to transition into a gas form.
The increased force pushes the boiling point away from where vaporization commonly occurs, requiring more energy to convert the liquid to a gas.
On the other hand, reducing pressure around a liquid will cause its boiling point to drop, allowing it to turn into a gas more quickly.
4. Intermolecular forces
These forces, which can be either attractive or repulsive, dictate how closely molecules approach each other and the strength of their interactions.
Stronger intermolecular forces will make it more difficult for molecules to leave the liquid phase, thus lowering their rate of vaporization.
On the other hand, weaker intermolecular forces facilitate molecule motion, increasing the ease with which molecules can break free from intermolecular bonds and enter a gaseous state.
Real-Life Applications of Liquid Changing to Gas (Vaporization)
Vaporization plays a critical role in numerous real-life applications. Cooling systems, for example, rely on vaporization to keep temperatures within an ideal range. In this system, the liquid is converted into a gas, causing a cooling effect as it absorbs heat energy from its surroundings.
Distillation processes also use vaporization to purify liquids by separating impurities with different boiling points. Boiling the liquid causes it to break into its constituent components – some of which turn into a gas and are drawn off, leaving only the desired result behind.
Vaporization has further been harnessed in fuel production. By heating oil or other hydrocarbons, they can be turned into a gaseous form that can be used as fuel in vehicles and other power sources.
Finally, vaporization is an integral part of the water cycle. Water evaporates from the surface of oceans and lakes, carrying it into the atmosphere and allowing it to travel around. This process relies upon liquid water turning into a gas before being brought back to earth in the form of rain or snowfall.
Other Related Phenomena
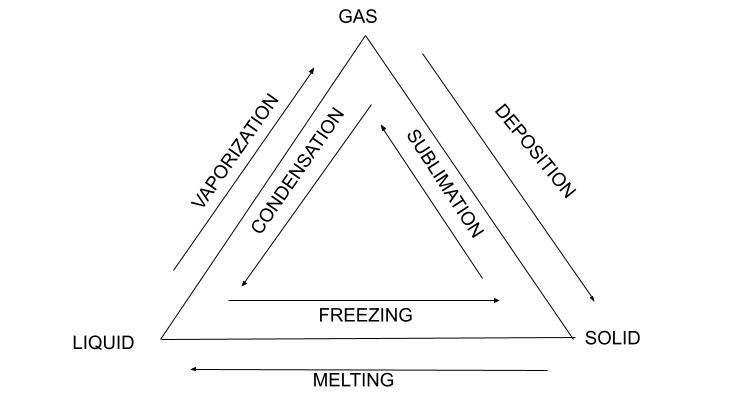
- Sublimation: conversion of a solid substance directly into a gas without melting into a liquid first.
- Melting: the process by which solid substances turn into liquids.
- Deposition: conversion of gas to solid.
- Condensation: the reverse vaporization process in which vapor is converted back to a liquid by cooling or pressure.
- Freezing: changing a liquid to a solid when cooled below freezing point.
FAQ
What is the difference between evaporation and boiling?
Evaporation is when a liquid changes to a gas at the surface of the liquid, while boiling is when a liquid changes to gas throughout the entire volume of the liquid.
Boiling occurs when the vapor pressure of a liquid equals the atmospheric pressure, while evaporation occurs at any temperature.
Can liquids evaporate at any temperature?
Yes, liquids can evaporate at any temperature. However, the evaporation rate depends on the temperature and the properties of the liquid, such as its vapor pressure and surface area. Higher temperatures and larger surface areas typically result in faster evaporation rates.
Summary
In summary, when a liquid changes to a gas, it is called vaporization. This process can happen through two main types of vaporization: evaporation and boiling.
Evaporation happens when a liquid turns into a gas on the surface of the liquid. On the other hand, boiling happens when a liquid is heated to its boiling point and turns into a gas throughout the liquid.
Both types of vaporization are affected by factors such as temperature, pressure, intermolecular forces, and surface area. Understanding vaporization is essential in many scientific fields and practical applications, such as the water cycle, cooking, and power generation.
For more exciting science articles, explore Biotrux.
Thanks for reading.